
DISCLAIMER: I'm not a chemist. I did well in high school chemistry, and I've furthered my education a bit on the side since then, but there is a chance I've made mistakes here that a proper organic chemists would wince at. This page isn't meant to be an absolute information resource. It's meant to try dispel some of the confusion around complex chemical names.
What I'm going to do on this page is take a drug used in treatment of some kinds of cancer and explain its full chemical name and show how it got the name. People who get spooked reading the side of a soda can can get utterly horrified with some of these drug names. I'd just like to show that these long and drawn out names have some reason for existing, and aren't just something that some nutty scientist thought up to confuse people.
I'm going to use a lot of long words from here in. Many of them are simple combinations of a start and an end, which I've put in [brackets]. I might also include short reminders where they actually occur in the text.
The words include the following:
Chemotherapy drugs are all - to the best of my knowledge - cytotoxins. While anything with the word "toxin" in it sounds dangerous, cytotoxins come in both good and bad varieties. Certain animal venoms are neurotoxins, which is a kind of cytotoxin. Yet your own immune system makes use of cytotoxins as well: bacteria and such are destroyed through the action of cytotoxins that kill bacteria but not human cells. The idea behind chemo being to use cytotoxins that are deadlier to cancer cells than they are to normal cells. However, normal cells often get caught in the 'crossfire', since cancer cytotoxins (antineoplastics) often attack cells that multiply rapidly. Unfortunately, cells like bone marrow and hair follicles also multiply rapidly. (And a lot of chemo patients lose their hair, and some require bone marrow donations or blood transfusions - blood cells are manufactured in bone marrow.)
Non-chemo drugs include things like hormone drugs (since some cancers feed on hormones, reducing the levels of those hormones will help 'starve' the cancer and slow its growth) and drugs to help rebuild the body.
The example drug I'm using today is trademarked as Zometa. Its generic name is zoledronic acid. Its chemical name is (1-hydroxy-2-imidazole-1-yl-phosphonoethyl) bisphosphonic acid monohydrate. It's a drug that helps stop bones from degenerating. Certain bone cancers cause a person's osteoclasts (which destroy bone) to grow and act faster. Normally the osteoclasts work in balance with the osteoblasts (which make bone), helping repair fractures and destroy old and weakened bone. (I've heard various statistics saying that a person goes through roughly three skeletons in their lifetime, with old bone slowly being replaced all over the body.)
But the cancer causes the osteoclasts to work faster than the osteoblasts, leading to bone degeneration. Eventually, bone pain sets in, moving becomes difficult, and bones fracture under even minor strain.
There are certain drugs, like zoledronic acid and pamidronate (also known as APD, trademarked Aredia, chemically named 'aminohydroxypropylidene bisphosphonate') which help bring the osteoclasts back in line. (Both of these drugs are considered 'bisphosphonates', more on that later.) While the body can't rebuild areas of bone that were utterly destroyed it can at least strengthen the remaining areas. (Our bodies don't do well with rebuilding, that's why we have scar tissue. The body incorrectly fills in the missing area. You'll notice that, unlike certain other animals, we can't regenerate lost limbs except in certain rare situations when some people grow back the tip of a finger.)
There is another bisphosphonate which you might have heard of in television or print advertisements. It's sold as a way for osteoporosis sufferers to rebuild their bones. It's called Fosamax, also known as alendronate sodium. Its chemical name is 4-amino-1-hydroxybutylidene 1,1-bisphosphonate (or sometimes 4-amino-1-hydroxybutylidene-1,1-bisphosphonic acid monosodium salt trihydrate).
Looks like something out of the opium-fried mind of a suburb road planner, doesn't it?
I'll explain it one step at a time.
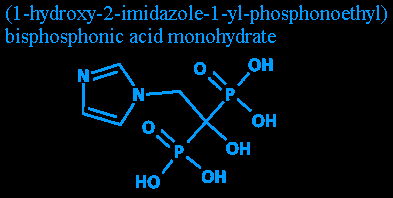 Step one: Reading the diagram. (NOTE: If you don't know a bit of chemistry, you might get a bit lost in here. I'll try bring you up to speed, though.)
Step one: Reading the diagram. (NOTE: If you don't know a bit of chemistry, you might get a bit lost in here. I'll try bring you up to speed, though.)
Everything's made up of atoms, which are usually found as molecules. Two oxygen atoms form an oxygen molecule [O2], two hydrogens and an oxygen form a water molecule [H2O], etc. Different atoms can connect to different numbers of other atoms (the connections are called 'bonds'). While there's over a hundred different kinds of atoms, we're concerned with only a few.
If two letters are slammed up against each other, like the OHs and the HO, they're bonded just like if there was a line. Oh, and there's no difference between OH and HO. They're both an oxygen with a hydrogen attached. It's just so it doesn't look like it's the hydrogen bonded to the phosphorus atom.
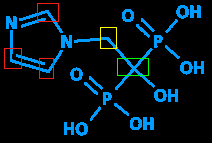 But back to the missing carbon. Carbon is essential to the field of organic chemistry - in fact, organic chemistry is by definition all about carbon and things connected to carbon - since it can form long chains and thus make lots of different chemicals, each with different length chains or different things attached to the chains. It's so essential that it's not even written in, to save space. See all those junctures with no letter? (The ones I've put colored rectangles around.) All those are carbons.
But back to the missing carbon. Carbon is essential to the field of organic chemistry - in fact, organic chemistry is by definition all about carbon and things connected to carbon - since it can form long chains and thus make lots of different chemicals, each with different length chains or different things attached to the chains. It's so essential that it's not even written in, to save space. See all those junctures with no letter? (The ones I've put colored rectangles around.) All those are carbons.
Another (partially) missing letter is H (hydrogen). Since hydrogen is a common atom and often found in the company of carbon (hence the term 'hydrocarbon' - you fill your car's gas tank with a host of hydrocarbons and other chemicals on a regular basis) it's omitted when attached to a carbon. Since carbons always have four bonds, the missing bonds are assumed to be hydrogens. In the example above, the red rectangles each have three visible bonds; the fourth on each is a hydrogen. The yellow rectangle has two visible bonds; the other two are hydrogens. And the green one, having four visible bonds, isn't attached to any hydrogens.
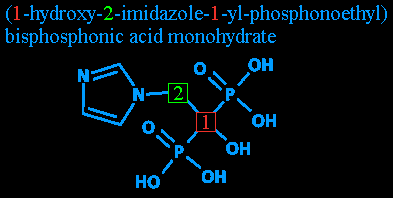 Step two: All those numbers! Despite the oddity of having numbers in a name, they're nothing to worry about. Complex organic compounds need some way of figuring out what's attached where. Since, as I mentioned above, they all have a long carbon chain, the chain's carbon 'links' are numbered.
Step two: All those numbers! Despite the oddity of having numbers in a name, they're nothing to worry about. Complex organic compounds need some way of figuring out what's attached where. Since, as I mentioned above, they all have a long carbon chain, the chain's carbon 'links' are numbered.
I can't remember how they choose which end to start with (been years since my last chemistry class). Some say it has something to do with starting from where the most (or largest) bunches of atoms are attached, some say it's so the numbers will be as low as possible. Either way turns out the same here, so I'm not worried about specifics.
So, to denote where on the chain something is attached, they simply use the number of the carbon it's attached to.
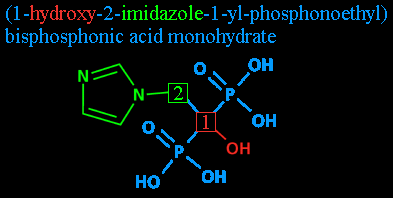 Step three: The radicals: 1-hydroxy and 2-imidazole. Radicals are groups of atoms that are ready to be bonded to something else. Here, I'm also using it to refer to the groups after they've bonded.
Step three: The radicals: 1-hydroxy and 2-imidazole. Radicals are groups of atoms that are ready to be bonded to something else. Here, I'm also using it to refer to the groups after they've bonded.
Attached to carbon #1 is a hydroxyl radical - basically, a water molecule that's lost one of its hydrogens. It gets its name from its components, hydrogen and oxygen, plus a yl is added to show that it's a radical. This leaves you with 'hydrooxyyl', with the double O and double Y clipped out. (Either to follow proper rules of grammar or to make it look less like something you'd hear in a Spanish-language football broadcast: 'GOOOOOOAAAAAALLLLLL!'.)
Since it's not in radical form here, it's just plain old 'hydroxy'.
Attached to carbon #2 is imidazole. That's the pentagonal thing with the nitrogens and the carbons and the double bonds. You'll note that since the imidazole is a radical unto itself, and because there's a nitrogen in the way, the carbons of the imidazole loop don't count towards the carbon chain's numbering.
 (Image repeated for convenience.)
(Image repeated for convenience.)
Step four: Radicals redux: 1-yl. 1-yl? As I mentioned before, the 'yl' denotes a radical. Here, it's being used (as far as I can tell - like I said, my chemistry is a bit rusty) to note that whatever else is mentioned after this is attached at carbon #1. After all, nothing is attached to carbon #2 except two unimportant hydrogens and the imidazole that was already pointed out.
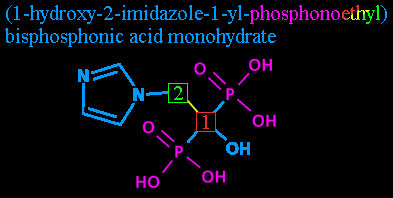 Step five: Phosphonoethyl. This we'll break into two parts.
Step five: Phosphonoethyl. This we'll break into two parts.
'Phosphono-' is a chemical prefix for those groups I've colored purple, the P(=O)(OH)2 groups. Each one is a phosphorus atom, double bonded to an oxygen atom and single-bonded to two hydroxyls. The fifth bond leads back to whatever comes next...
... and in this case that's the 'ethyl'. Notice that despite all this fancy numbering we haven't even listed how many carbons there are? As it stands now, there could be a 50-carbon chain growing off that carbon #2 and as long as it didn't have anything but hydrogens attached to it, we'd never hear about it. Now is time to change that.
'Ethyl' shows that the chain is two carbons long. A two-carbon chain without anything other than hydrogens is called ethane. One carbon with four hydrogens attached is methane. Accoring to standard nomenclature (rules of naming), the chains from one-carbon to twelve-carbon are called: methane, ethane, propane, butane, pentane, hexane, heptane, octane, nonane, decane, undecane, dodecane. (Some of those should look familiar even to laymen.)
Again, the removal of the -ane (which lets you know it's nothing but a chain of carbons, all single bonds, with hydrogens attached) and the addition of the -yl suffix shows it's a radical. As in, it's not attached to just hydrogens anymore. (You might be familiar with ethanol, also called ethyl alcohol. It's an ethane with one of the hydrogens replaced with a hydroxyl. It's also what gets a person drunk.)
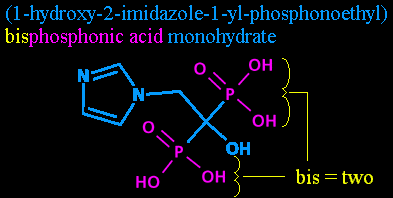 Step six: Bisphosphonic acid. While we've already mentioned the phosphorous atoms and their attendant hydroxyls with the phosphono- in phosphonoethyl, we didn't say how many there were! So it's time to mention it again, outside the parentheses. (Parentheses are used to help provide clarity in complex names, and show what's attached where.) Adding the 'bisphosphon-' also helps point out that this is in a class of chemicals called the bisphosphonates, as mentioned previously.
Step six: Bisphosphonic acid. While we've already mentioned the phosphorous atoms and their attendant hydroxyls with the phosphono- in phosphonoethyl, we didn't say how many there were! So it's time to mention it again, outside the parentheses. (Parentheses are used to help provide clarity in complex names, and show what's attached where.) Adding the 'bisphosphon-' also helps point out that this is in a class of chemicals called the bisphosphonates, as mentioned previously.
'Bis-' is just another prefix for two. I'm told it's sometimes seen in normal English, taking the place of 'bi-' in front of certain letters, but I'm temporarily at a loss for an example.
And 'phosphonic acid' is just another way of describing the P(=O)(OH)2 groups.
So, while '1-yl-phosphonoethyl' told us where the P(=O)(OH)2 was on the carbon chain, 'bisphosphonic acid' tells us there's two of them there, and points out that it's an acid and a bisphosphonate at the same time.
Oh, and not all acids are as caustic as something from an Alien movie. For instance, DNA is deoxyribonucleic acid, and people and animals are full of it.
By the same token, not all caustic things are acids. Chlorine bleach has an active substance called sodium hypochlorite (NaOCl). It's an alkali (a base); just the opposite of an acid yet very caustic. And, like all bases, it reacts with acids easily. But unlike benign childhood experiments with vinegar (acid) and baking soda (base), bleach gives off dangerous chlorine gas in the process. That's why bleach bottles often have a big warning not to mix it with anything not made for mixing with bleach, especially anything acidic.
Step seven: Monohydrate. That just means that one [mono-] water molecule [-hydrate] tends to hang on to the main zoledronic acid molecule. (At the 1-hydroxyl radical.) Lots of things collect water molecules like that. Some, like sodium hydroxide, collect so many that if left out in the air, moisture from the air will condense on them until there's a tiny puddle of water with the chemical diluted in it.
 Well, that wraps up the naming of this complex compound. I hope that the logic of how these things are named makes a little more sense than it did before.
Well, that wraps up the naming of this complex compound. I hope that the logic of how these things are named makes a little more sense than it did before.But before I go, I'd like to mention one thing to better educate you in chemistry. Often, you'll hear demagogues screaming about how some chemical is "one atom away" from some dangerous compound and is therefore itself dangerous. This is nothing but fearmongering. It would be like claiming that, for example, because your cousin murdered someone you should be locked up, since you're just one branch of the family tree away and are therefore just as dangerous. This logic doesn't hold water.
Allow me to give some examples of benign substances that are "one atom away" from a dangerous chemical.
Water is H2O. Or, structurally, H-O-H.
Hydrogen peroxide is H2O2, or H-O-O-H.
Would you want to drink peroxide? And remember, the stuff from the store is diluted. At over 8% concentration, it's corrosive. At high concentration, it can become explosive if exposed to light, heat, rust, or countless other things. Do that to pure water and all you get is warm rusty water.
Table salt is NaCl.
Sodium hypochlorite is NaOCl.
Yeah, chlorine bleach isn't that far off of table salt. In fact, I read that it can be made out of salt water and an electric current, assuming you have the proper anode/cathode setup to run the current through. (You'll note that the sidebar features a smaller bond marking for the Na. It's a special kind of bond called an ionic bond, and I think that's how it's shown. All the other bonds we've been dealing with are covalent bonds. In an ionic bond, one atom grabs an electron from the other and keeps it. In a covalent bond, the two atoms share a few electrons.)
And then there's uracil. It's needed by our body to manufacture RNA, a cousin to DNA that's a vital component to our well-being. Meanwhile, swap the hydrogen on ring atom #5 for a fluorine and you get 5-Fluorouracil, (un)affectionately and appropriately known as "5-FU". (Unlike above, here the carbons and nitrogens are both counted in the numbering, since we're just dealing with one ring and nothing else.)
It's a chemotherapy drug. It kills cells in the body (cytotoxic), including cancer cells (antineoplastic). It stops new cells from growing (antimetabolite). Works great on cancer, but does a number on the body as well. Often given as part of a "cocktail" of multiple chemo drugs, like fellow antimetabolite methotrexate, immunosuppressant/cytotoxin cyclophosphamide, or antimetabolite doxorubicin.
And these aren't the only 'one-off' molecules out there.
(Update, November 7, 2004 AD: The sidebar reference to measuring chemo drugs might be slightly off, since the information is a bit old. They're still extremely careful, though.)
Chemistry is an odd thing; biochemistry even more so. Our bodies are a complex mix of chemicals, some unique to ourselves. While sometimes similar chemicals do similar things, more often than not they wildly vary in their behaviors. Our bodies change the chemicals in ways that are sometimes barely understood. For instance, one drug mentioned above (cyclophosphamide) isn't a cytotoxin itself. But (as this site explains) after one trip through the body, some of it has been converted to "phosphoramide mustard and acrolein, both highly cytotoxic".
There's a lot of things in this world that might seem scary, but aren't. There's also a lot that look harmless, but aren't. Worrying about something with a threatening-sounding name is a futile act - it's not the name that hurts you. It might help to remember that the next time a person is looking at the name of a chemical that worries them. Study the chemical and learn its behavior. You can't judge a book by its cover, and you can't judge a chemical (or a person, for that matter) by its name.
Be well,
The Archon
February 8, 2004 AD